About
Hepatic de novo lipogenesis (DNL) is the synthesis of fatty acids in the liver from their metabolic precursor acetyl-CoA and is an important pathway reflecting metabolic health. Abnormal regulation of hepatic DNL has been observed in insulin resistance, type 2 diabetes and nonalcoholic fatty liver disease (NAFLD) (1,2), which makes it an appealing target for the treatment of metabolic diseases. Quantifying DNL using stable isotopes, therefore, is a valuable pharmacodynamic (PD) marker in early clinical trials. ProSciento scientists have extensive experience in the use of stable-isotope tracer methodologies, including the measurement of DNL in phase 1 and phase 2 clinical trials.

Recommended Materials
Posters
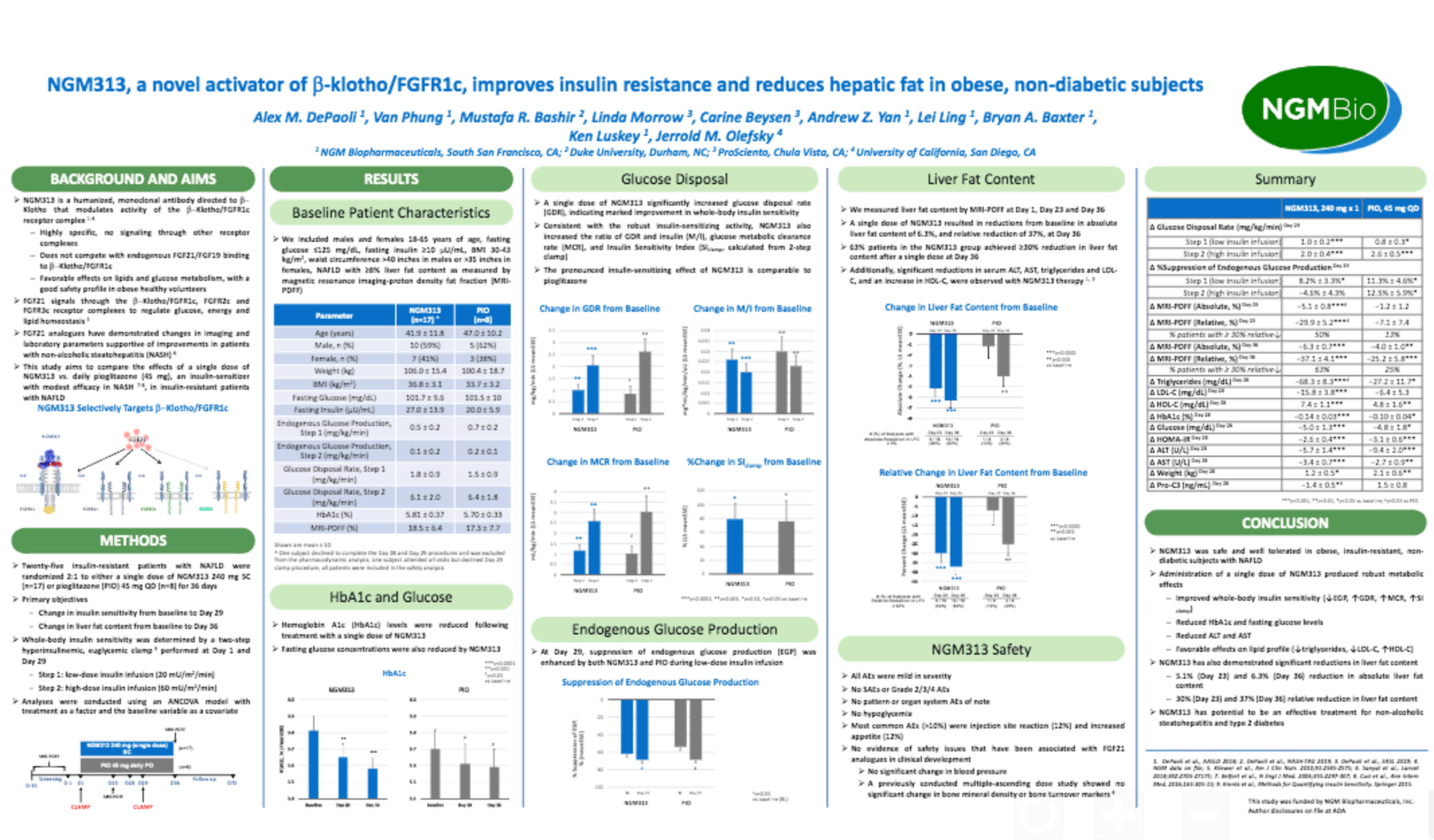
NGM313, a Novel Activator of β-Klotho/FGFR1c, Improves Insulin Resistance and Reduces Hepatic Fat in Obese, Nondiabetic Subjects

Posters
NGM313, a Novel Activator of β-Klotho/FGFR1c, Improves Insulin Resistance and Reduces Hepatic Fat in Obese, Nondiabetic Subjects

Articles
Adipose tissue measurement in clinical research for obesity, type 2 diabetes and NAFLD/NASH
Related Solutions
Specialized Methods




